Cracking the Code of Pain | Ep. 717
How NaV Channels Are Finally Delivering on Their Drug Discovery Promise
Hello Avatar! Welcome back for another week of biotech analysis. Today is Sunday, which means this is our Building Biotech newsletter that is focused on discussing biopharma strategy topics. Today we will dive into one of drug development’s most stubborn challenges, treating pain without addiction, sedation, or trade-offs. For years, voltage-gated sodium channels tempted pharma with genetic clues but delivered mostly disappointment. Now, a new generation of selective NaV1.8 inhibitors is reshaping the field, with Vertex’s suzetrigine leading the charge. Could this finally be the opioid alternative we’ve been waiting for?
We are now publishing 7x per week according to the following cadence:
Mondays: Stocks
Tuesdays: Biotech
Wednesdays: Podcast
Thursdays: Markets
Fridays: News
Saturdays: Podcast
Sundays: Strategy
We are also publishing unique content on X - be sure to follow up if you are not already @BowTiedBiotech. And to check-out the archive of our work on X you can find it on our website at: BowtiedBiotech.subtack.com/x-articles.
SUBSCRIBE TO PODCAST HERE:
Please help spread the work by subscribing and hitting the share button if you are enjoying our bi-weekly newsletters!
Enough shilling for the day, lots to cover this week, let's get started!
Introduction: NaVs, the Gatekeepers of Excitability
Pain remains one of the most intractable problems in medicine.
While opioids offer effective relief, their side effects have fueled a national addiction crisis, underscoring the need for safer, non-addictive alternatives. Voltage-gated sodium (NaV) channels have emerged as a compelling solution. These ion channels drive the electrical activity that underlies pain signaling, and several isoforms, including NaV1.7 and NaV1.8, are selectively expressed in peripheral sensory neurons. With the right level of specificity, they offer the possibility of pain relief without central nervous system side effects.
After decades of setbacks, clinical success with Vertex’s suzetrigine, a highly selective NaV1.8 inhibitor, has brought the field into sharp focus. Today we explore why NaV channels are such attractive targets, how suzetrigine overcame past challenges, and what this means for the future of pain therapeutics.
Why NaVs Are Compelling Drug Targets
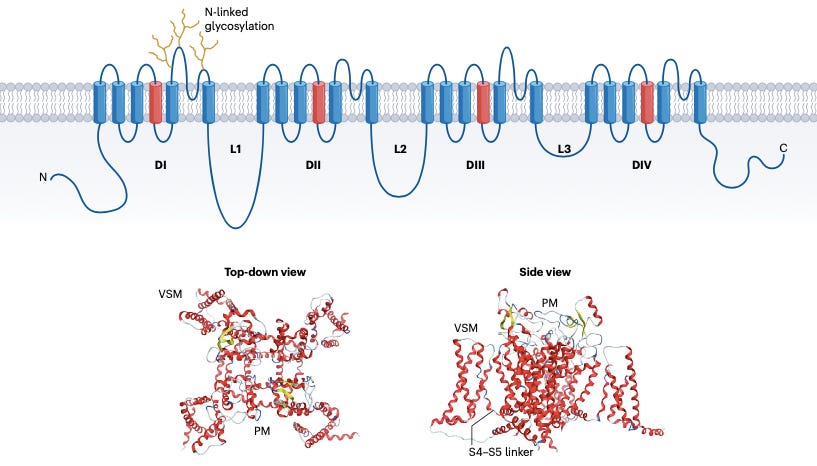
NaV channels are essential for generating action potentials in excitable cells. In sensory neurons, they act as amplifiers of pain. NaV1.7 sets the firing threshold, NaV1.8 carries the action potential current during high-frequency firing, and NaV1.9 contributes to persistent excitability during chronic inflammation. This triad makes NaV1.7, 1.8, and 1.9 especially attractive for analgesia.
What separates these targets from other ion channels is their validated human genetic relevance. Individuals with SCN9A loss-of-function mutations (NaV1.7) are pain-free, while gain-of-function mutations in the same gene cause severe pain syndromes. Mutations in NaV1.8 (SCN10A) are also linked to painful neuropathies.
NaV1.8 stands out because its expression is largely restricted to nociceptors. This minimizes the risk of off-target effects in the brain or heart, which complicate inhibition of other isoforms like NaV1.5 (cardiac) or NaV1.1 (CNS).
These characteristics position NaV1.8 as a “Goldilocks” target, high impact, low collateral damage.
BTBiotech Note: While NaV1.8 is mostly expressed in nociceptors, low-level expression in cardiac tissue has been reported. Long-term inhibition might still carry unforeseen safety liabilities, especially in frail or elderly populations.
Technical Approaches to Drugging NaV Channels
Keep reading with a 7-day free trial
Subscribe to BowTiedBiotech to keep reading this post and get 7 days of free access to the full post archives.