Welcome to the 20 NEW Biotech Innovators who have joined us this month! If you haven’t subscribed, join the 1,196 researchers, investors, operators, academics, clinicians and entrepreneurs by subscribing here:
Hello Avatar! Welcome back for another week of biotech analysis. With the always popular American Society for Gene and Cell Therapy (ASGCT) concluding this week we thought it would be fun to summarize the event and highlight some of the more interesting (in our opinion) presentations. We will also outline a few key themes that rose to the surface throughout the week. All in all, what a time to be involved in cell and gene therapy, it feels like the industry is truly at an inflection point and just beginning to scratch the surface toward breakthrough products. WARNING: today’s writeup will be long and technical - if you enjoy that you are in for a treat! For those of you not from a technical background, consider this an overview of the technology platforms that are up next which we are most excited about.
Please help spread the work by subscribing and hitting the share button if you are enjoying our bi-weekly newsletters!
As a reminder, the purpose of the BowTiedBiotech substack is two-fold. Primarily, we aim to provide our scientist audience the tools to build a biotech company and ultimately translate their ideas into medicines for patients. Secondarily, biotech investors may find this substack useful as we will be providing weekly market updates of the public AND private markets as well as heavily leveraging current financing events as teaching examples.
We are now operating under a hybrid subscription model where the Sunday and Monday updates have moved under the paywall - a BIG THANK YOU to those that have joined. The Thursday market update will continue to remain FREE. The more forward looking content, the insider insights, and market data you need to really assess what is happening in the world of biotech will shift to Sundays. Monday’s we focus on public biotech research for those of you who play the markets. And of course all subscribers get access to our growing library of everything they don’t teach you in school about the biotech industry (176 articles to-date).
Enough shilling for the day, lots to cover this week, let's get started!
ASGCT OVERVIEW
By far, our favorite conference of the year is the American Society for Gene and Cell Therapy, also known as ASGCT. This week the who is who of the C> world came together in Los Angeles to share their latest updates.
From our perspective 7 key themes emerge from the meeting: 1)Viral Delivery 2) Non-Viral Delivery 3)Editing 4)Commercial C> 5) Cell Therapy 6) HSCs 7) Bcells
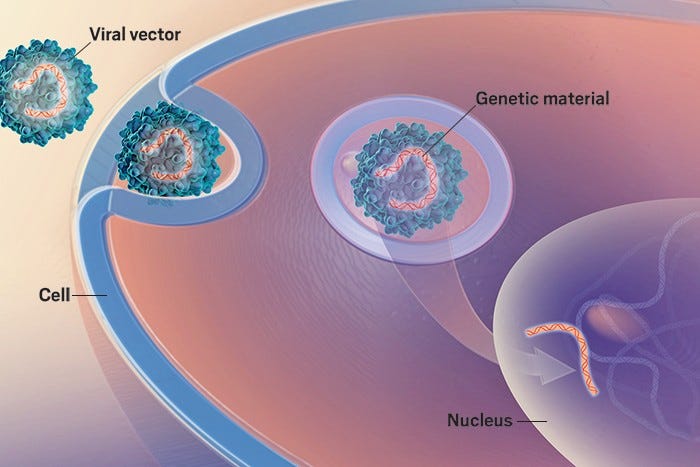
VIRAL DELIVERY
Viral delivery (particularly AAV) remains king in terms of volume of work shared. To-date virus have been an excellent modality with a proven clinical track record for delivering DNA into the nucleus of cells. Despite success in areas such as hemophilia, a number of challenges have emerged including manufacturability, re-doseability, durability, delivery/“beyond liver tropism”, and safety associated with over-expression strategies. Below we share a handful of interesting abstracts focused on the delivery problem.
The work below from the Sabin group at Regeneron describes a platform that has been successfully used to retarget multiple AAV serotypes to specific cell types in vitro and in vivo, with enhanced on-target delivery and liver detargeting compared to standard wildtype serotypes. The platform holds promise for accelerating the development of effective gene therapies for a variety of human diseases.
The below work from Samai et. al, from Regeneron describes a species-agnostic AAV retargeting approach using rational capsid engineering and monoclonal antibodies to redirect viral particles to skeletal muscles, achieving enhanced delivery in mouse models of disease such as Duchenne muscular dystrophy. The platform was demonstrated to be cross-species translational, with similar results observed in rodents and cynomolgous monkeys.
The work below from Firnberg et. al, from REGENEX describe chimeric AAV vectors that incorporate designed ankyrin repeat proteins (DARPins) engineered to target specific cell membrane receptors. They optimized multiple parameters of the DARPin-AAV fusion construct and production and found that DARPin insertions in VR-IV of VP1 could mediate >25-fold increased transduction of HEK293 cells over-expressing the target receptor compared to the unmodified vector.
NON-VIRAL DELIVERY
As expected, drug delivery remains a central focus in the field of medicine. In the past year, the abundance of abstracts on novel AAV capsids reflected the saturation of interest in this delivery system. However, in 2023, the focus has expanded to include a more diverse range of technologies. Among these are RNPs, ultrasound/electroporation, mAb-conjugated AAVs, and kidney tropism.
RNPs, or ribonucleoproteins, are complexes of RNA and proteins that play critical roles in gene regulation. In the context of drug delivery, RNPs can be engineered to deliver specific RNA molecules to target cells. This technology holds promise for the treatment of genetic diseases, such as cystic fibrosis.
Keep reading with a 7-day free trial
Subscribe to BowTiedBiotech to keep reading this post and get 7 days of free access to the full post archives.