Hello Avatar! Welcome back for another week of biotech analysis. Today is Sunday, which means this is our Building Biotech newsletter that is focused on discussing biopharma strategy topics. Cancer therapy has evolved significantly over the past century, with advancements aimed at increasing the specificity and effectiveness of treatments while minimizing damage to normal cells. One of the most promising developments in this field is the use of monoclonal antibodies (mAbs) and their various engineered formats, including ADCs, and T cell engagers. Today we are going to discuss the evolution of this space, each modalities mechanism of action, clinical applications, and ongoing research to enhance their efficacy and reduce toxicity.
If you're not subbed yet click the link below. Every Thursday we are out with our FREE public/private biotech market update. Sundays are the days we focus on forward looking strategy. Monday’s are for public equity research. Tomorrow we will focus on Rezolute which is set to receive a crucial decision from the FDA regarding the partial clinical hold on RZ358 for Congenital Hyperinsulinism (CHI). With options pricing indicating an astounding 160% volatility, the market is bracing for significant movement.
Please help spread the work by subscribing and hitting the share button if you are enjoying our bi-weekly newsletters!
Enough shilling for the day, lots to cover this week, let's get started!
ANTIBODY GENERATION AND ENGINEERING
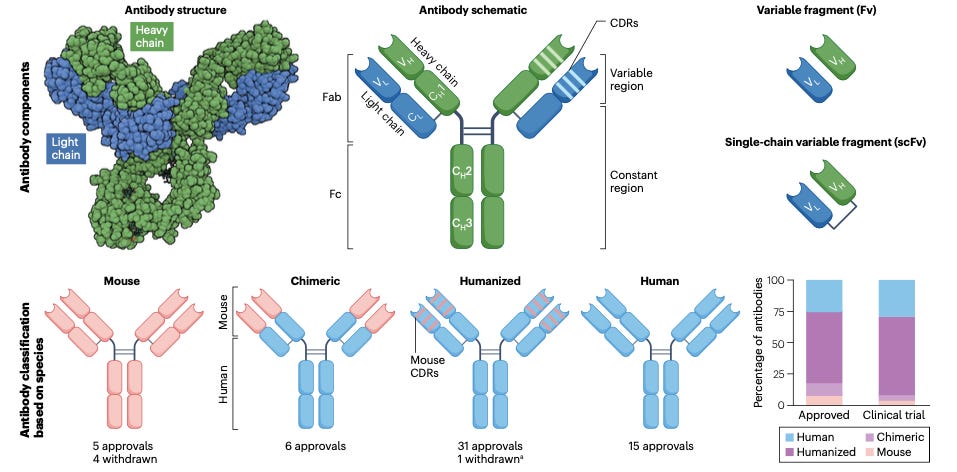
The journey of therapeutic antibodies began with the development of hybridoma technology in the 1970s, allowing the production of specific mouse monoclonal antibodies targeting human antigens. However, the clinical application of these mouse antibodies was limited due to immune responses against them, known as human anti-mouse antibody (HAMA) responses. To overcome this, researchers developed chimeric antibodies by grafting mouse variable regions onto human constant regions, and further advancements led to the humanization of antibodies, retaining only the complementarity-determining regions (CDRs) of mouse antibodies in a human antibody framework.
In the 1990s, fully human antibodies were developed using transgenic mice and phage display technologies. These advancements have led to the approval of several fully human antibodies for cancer therapy, including notable examples such as daratumumab (anti-CD38) and nivolumab (anti-PD1) shown below. The generation of fully human antibodies significantly reduced the risk of immunogenicity and adverse reactions in patients, thus enhancing their therapeutic potential.
ANTIBODY BODY FORMATS IN CANCER THERAPY
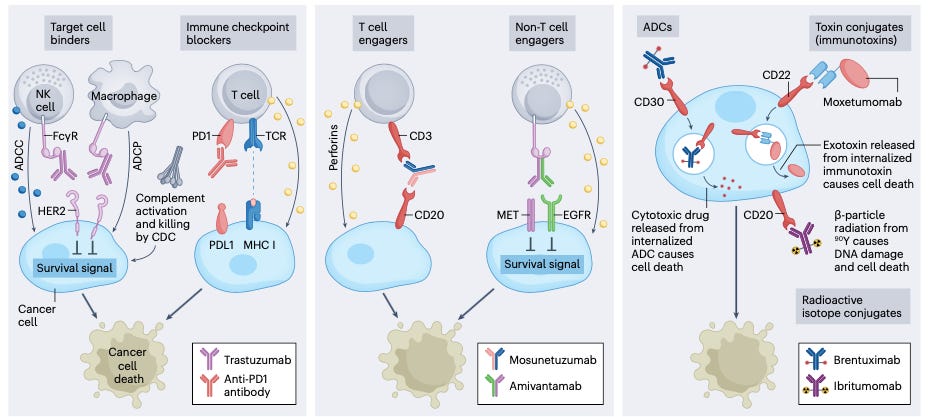
The landscape of antibody-based cancer therapies is diverse, encompassing several formats that leverage the unique properties of antibodies to target and eliminate cancer cells. These formats can be broadly categorized into monospecific antibodies, bispecific antibodies, and drug-conjugated antibodies, each with distinct mechanisms of action and therapeutic applications.
These diverse mechanisms highlight the strategic innovations in antibody-based cancer therapies, offering highly specific and effective treatments tailored to target and destroy cancer cells while minimizing harm to healthy tissues.
Monospecific Antibodies
Keep reading with a 7-day free trial
Subscribe to BowTiedBiotech to keep reading this post and get 7 days of free access to the full post archives.