Epoch 38: SURVEY OF THE CRISPR CLINICAL LANDSCAPE
Challenges, Recent Advancements, Future Directions
Hello Avatar! Welcome back for another week of biotech analysis. Today is Sunday, which means this is our Building Biotech newsletter that is focused on discussing biopharma strategy topics. Today, we’re going to dive into the evolving landscape of CRISPR-based therapies, including the milestone approval of Casgevy and the exciting advancements in base and prime editing. We'll also explore emerging technologies like chemically inducible systems and epigenetic editing, which are broadening the scope of gene therapy. As these innovations continue to shape personalized medicine, we’ll discuss their potential to provide transformative treatments for genetic and epigenetic diseases. Finally, we’ll address the critical technical, safety, and ethical challenges that must be overcome to unlock the full promise of gene editing.
If you're not subbed yet click the link below. Every Thursday we are out with our FREE public/private biotech market update. Sundays are the days we focus on forward looking strategy. Monday’s are for public equity research. Tomorrow we will focus on Repare Therapeutics (RPTX) who is expected to provide results from the phase 1 MYTHIC study, which includes the combination of lunresertib (LUMINAS) and camonsertib (CAM-NI) for the treatment of platinum-resistant ovarian and endometrial cancer, in Q4 2024. With options pricing indicating an astounding 165% volatility, the market is bracing for significant movement.
Please help spread the work by subscribing and hitting the share button if you are enjoying our bi-weekly newsletters!
Enough shilling for the day, lots to cover this week, let's get started!
CRISPR TECHNOLOGY OVERVIEW
The CRISPR-Cas9 system has revolutionized the field of gene editing since its discovery. This technology, which stands for Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9, allows scientists to make precise, directed changes to the DNA of living organisms.
The approval of Casgevy by the U.S. Food and Drug Administration (FDA) marked a significant milestone in the field of gene therapy. Casgevy, the first CRISPR-based drug approved for human use, offers a groundbreaking treatment for sickle cell anemia by permanently altering the gene responsible for the disease. This approval signifies the beginning of a new era in medicine, where CRISPR technology can be used to treat a wide range of genetic disorders.
Today we aim to discuss the advancements, challenges, and future prospects of CRISPR-based therapies. By examining the mechanisms of CRISPR, case studies like Casgevy, and ongoing trials, we can understand the transformative potential of this technology in treating genetic disorders. For those looking to read more on this topic we would point you to a recent publication from Lei Qi in Nature which you can access here. Finally on the backend of this analysis we will conclude with an “appendix” of upcoming catalysts as well as a landscape of the private and public markets.
CRISPR-CAS9 101
CRISPR-Cas9 is a groundbreaking technology that has revolutionized the field of genetic engineering. At its core, CRISPR-Cas9 allows scientists to make precise changes to DNA, the molecule that carries the genetic instructions for all living organisms. Many are not aware that this system is derived from a natural defense mechanism found in bacteria, which use it to recognize and cut the DNA of invading viruses. By adapting this system for research, scientists are now able to edit the genomes of plants, animals, and even humans.
The CRISPR-Cas9 system consists of two key components: the Cas9 nuclease, a protein that cuts DNA, and a guide RNA (gRNA), which directs Cas9 to the specific location in the genome where a cut needs to be made. The gRNA contains a short sequence of RNA that matches the target DNA sequence, guiding the Cas9 protein to the exact spot. Once Cas9 introduces a double-strand break in the DNA, the cell’s natural repair processes take over. These repair processes can either introduce small mutations that disrupt the gene or allow scientists to insert new genetic material at the break site, creating opportunities to correct genetic defects, enhance traits, or study gene function.
This ability to precisely modify the genome has opened up new possibilities in medicine, agriculture, and basic research, making CRISPR-Cas9 one of the most powerful tools in modern biology.
CASE STUDY: CASGEVY FOR SICKLE CELL
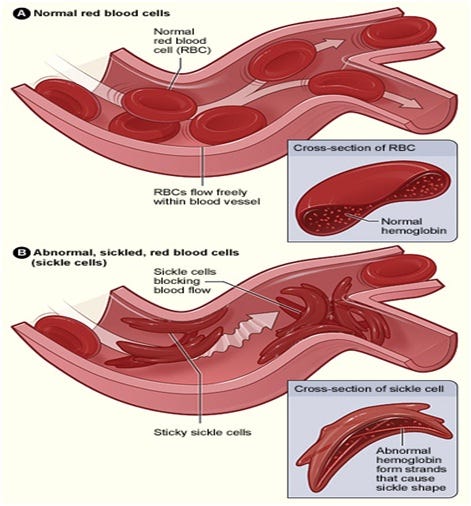
Sickle cell anemia is a hereditary blood disorder caused by a mutation in the hemoglobin beta gene (HBB), which is responsible for producing hemoglobin—a protein in red blood cells that carries oxygen throughout the body. In individuals with sickle cell anemia, the mutation causes the production of abnormal hemoglobin, known as hemoglobin S. This abnormal hemoglobin causes red blood cells to take on a rigid, sickle-like shape, which reduces their ability to carry oxygen and leads to blockages in blood vessels. These blockages result in painful episodes, organ damage, and other serious health complications.
https://www.1000sciencefairprojects.com/Biology/sickle-cell-anemia.php
Traditional treatments for sickle cell anemia, such as blood transfusions and bone marrow transplants, aim to manage symptoms or replace defective blood cells. However, these treatments come with significant limitations. Blood transfusions only provide temporary relief, and bone marrow transplants, which offer a potential cure, require a matched donor and carry risks of complications and rejection.
CasGevy represents a breakthrough in gene therapy for sickle cell anemia. Using the CRISPR-Cas9 system, CasGevy targets and repairs the underlying genetic mutation in the HBB gene. By editing the patient’s own cells, CasGevy allows for the production of healthy hemoglobin, potentially curing the disease at its source. This approach offers a more permanent solution, addressing the root cause of sickle cell anemia rather than just managing its symptoms.
The development of gene therapies like CasGevy marks a significant advancement in the treatment of genetic disorders, giving hope to those living with conditions like sickle cell anemia that have historically been difficult to cure.
Casgevy offers a novel solution by using CRISPR-Cas9 to disrupt the BCL11A gene, a suppressor of fetal hemoglobin production. This ex vivo process involves editing bone marrow cells outside the patient’s body to reactivate the production of fetal hemoglobin, which can compensate for the defective β-globin.
The approval of Casgevy was based on successful clinical trials that demonstrated its efficacy in treating sickle cell anemia. Patients treated with Casgevy showed significant improvements, highlighting the potential of CRISPR-based therapies to provide long-lasting and potentially curative treatments for genetic disorders.
CRISPR IN THE CLINIC
CRISPR technology has advanced beyond its initial success in treating sickle cell anemia and has now entered human clinical trials for a variety of other genetic disorders. These trials include efforts to address conditions such as β-thalassemia, hereditary transthyretin amyloidosis, Duchenne muscular dystrophy, and cystic fibrosis, among others. By targeting and editing disease-causing genes, these trials aim to provide long-lasting and potentially curative treatments. In the following sections, we will explore a survey of these ongoing CRISPR clinical trials and the promising developments they represent.
For us, the key to CRISPR realizing its full potential will come in one or both of 2 forms. First, it is possible if we start to see consistent cures being delivered, pharma interest may again perk up and recognize value in these platforms. Second, as delivery technology matures, there will naturally be more opportunity to drug mechanisms associated with larger TAM disease. At the moment we have a commercial problem with the overall platform as revenue projections are misaligned with valuation expectations.
Next we will run through some of the disease areas where there is activity from CRISP therapeutic developers. Before going into detail, the table below highlights a range of ongoing gene editing trials, with most early programs relying on ex vivo approaches, where cells are edited outside the body and then reinfused. Examples include CRISPR Therapeutics' CAR T cell therapies for cancer and Fate Therapeutics' iPSC-derived therapies. These programs primarily focus on leveraging Cas9 nuclease for targeted gene disruption or insertion in the context of cancer and immune-related diseases.
We are now seeing the rise of in vivo editing, as demonstrated by Intellia's NTLA-2001 program, which uses Cas9 delivered via lipid nanoparticles (LNPs) to target the TTR gene in hATTR, a key milestone for in vivo gene therapy. In addition, novel editing approaches like epigenetic editing from Tune Therapeutics and base editing from Beam Therapeutics are emerging, expanding the gene-editing landscape beyond traditional CRISPR-Cas9 methods and into more sophisticated gene modulation technologies.
Keep reading with a 7-day free trial
Subscribe to BowTiedBiotech to keep reading this post and get 7 days of free access to the full post archives.