Welcome to the 103 NEW Biotech Innovators who have joined us this month! If you haven’t subscribed, join the 1,490 researchers, investors, operators, academics, clinicians and entrepreneurs by subscribing here:
Hello Avatar! Welcome back for another week of biotech analysis. This week was another historic one in the field of genetic medicine. On Friday, FDA approved the first ever CRISPR treatment for patients with beta thalassemia and sickle disease from CRISPR and Vertex. To build on this excitement we figured we would feature KOL commentary on what comes next. William and Blair recently organized a discussion on this topic that touched on the areas of pricing, delivery (beyond liver), and moving beyond rare disease. Many of these topics have already been discussed on BowtiedBiotech so it is nice to see our views align with leaders in the field.
If you're not subbed yet click the link below. Every Thursday we are out with our FREE public/private biotech market update. Sundays are the days we focus on forward looking strategy. Monday’s are for public equity research. We are accelerating tomorrow’s update to later today - we will focus on Editas ($EDIT) with their upcoming data from EDIT-301 for TDT and SCD. The options market projects a 20% move.
Please help spread the work by subscribing and hitting the share button if you are enjoying our bi-weekly newsletters!
As a reminder, the purpose of the BowTiedBiotech substack is two-fold. Primarily, we aim to provide our scientist audience the tools to build a biotech company and ultimately translate their ideas into medicines for patients. Secondarily, biotech investors may find this substack useful as we will be providing weekly market updates of the public AND private markets as well as heavily leveraging current financing events as teaching examples.
Enough shilling for the day, lots to cover this week, let's get started!
HOT TOPICS IN GENETIC MEDICINE
This week's newsletter is inspired by a William Blair event from a few months back: Innovator Series: Genetic Medicine. We thought the session was quite good and nicely covered the key issues at the forefront of genetic medicine today. The day featured a number of high profile speakers and since genetic medicine is such a hot topic on the twitter feed, we thought it was worth the time to cover for our readers.
The event was structured around 3 Key Themes, followed by deep dives into Neurodegeneration and Neuromuscular diseases.
Pricing
Delivery (beyond liver)
Large Indications
GENETIC MEDICINE AND DRUG PRICING
At the time of the event the CRISPR/Vertex approval had not yet occurred. Michael Heifetz, senior managing director at Pathway Healthcare, offered valuable insights into the unique regulatory considerations that may arise post-approval.
Testing the Limits of "Rare Disease": Unlike prior gene therapies targeting smaller patient populations under private insurance, SCD presents a unique challenge. Its large patient population, primarily covered by Medicaid, pushes the boundaries of the “rare disease” definition. This raises questions about how the Medicaid system will handle the potential influx of patients seeking these transformative therapies.
State Budgets Prepared, But Demand Uncertain: While concerns exist about potential budget strains on the Medicaid system, Heifetz believes state budgets are already preparing for this eventuality. However, he acknowledges the possibility that these preparations may underestimate the actual demand and financial impact of these therapies. Despite this, he remains optimistic that budget constraints will not become a major barrier to patient access.
Navigating Prior Authorization: Heifetz emphasizes the crucial role that physicians and patient advocacy groups will play in navigating the prior authorization process, ensuring patients receive timely access to these life-changing therapies.
Streamlining Reimbursement: As private and public health insurance payers gain experience with the long-term outcomes of genetic medicines, Heifetz anticipates a gradual streamlining of the reimbursement process, making access more efficient and equitable for all patients.
While not really drilling down into the specifics, this conversation highlighted the complex landscape that CRISPR/Vertex and bluebird's gene therapies for SCD face post-approval. While challenges exist, Heifetz's insights offer a reassuring perspective, suggesting that state preparedness, the dedication of healthcare professionals and patient advocates, and a learning curve for insurance payers will pave the way for successful access to these transformative therapies.
DELIVERY OF GENETIC MEDICINE (INCL BEYOND LIVER)
One of the biggest challenges facing genetic medicine is optimizing delivery and minimizing off-target effects. The liver (for better or worse) acts as a "sink" for many delivery vehicles, such as lipid nanoparticles (LNPs) and adeno-associated viruses (AAVs). Below we explore active strategies to redirect LNPs and AAVs beyond the liver.
Targeted LNP Approaches:
Passive targeting: Utilizing the natural properties of LNPs to accumulate in specific tissues based on their size, charge, and other characteristics.
Active targeting: Attaching ligands to the LNP surface that bind to specific receptors on target cells, directing delivery with greater precision.
Endogenous targeting: Exploiting endogenous molecules within the body to guide delivery to specific tissues or cells.
Promising LNP Developments:
ReCode Therapeutics: Their SORT LNPs incorporate a 5th lipid with the ability to deliver genetic payloads to the spleen and lung, currently undergoing clinical evaluation.
Generation Bio: Stealth LNPs combined with their novel iqDNA payloads offer a promising non-viral gene therapy alternative.
Targeted AAV Approaches:
Capsid Serotype Optimization: Several AAV companies, including Kriya, Myrtelle, Tenaya, and Rocket, focus on meticulously selecting and optimizing AAV capsid serotypes for high tropism towards their target cell populations, leading to reduced liver uptake and improved delivery efficiency.
These innovative approaches demonstrate the significant progress being made in overcoming delivery hurdles and de-targeting the liver in genetic medicine. By exploring diverse strategies such as novel LNP designs and selective AAV serotypes, researchers are paving the way for more precise and effective delivery of genetic therapies to various target tissues, ultimately leading to improved patient outcomes.
GENETIC MEDICINE FOR LARGE INDICATIONS
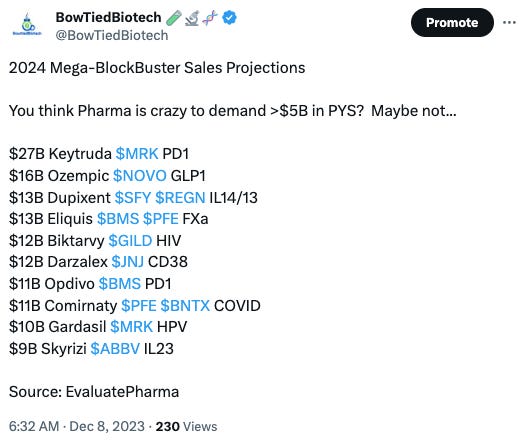
The days discussion then shifted to the potential for the application of genetic medicine across neuromuscular, CNS, and cardiovascular indications. This is noteworthy, because the initial proof of concept indications have been limited to smaller rare disease. As we have been hammering on the X timeline for better or worse these smaller peak year sales (PYS) potential are currently out of favor with pharma.
Neuromuscular Diseases:
Keep reading with a 7-day free trial
Subscribe to BowTiedBiotech to keep reading this post and get 7 days of free access to the full post archives.